
Brief introduction to the deployment of medical IT systems
Safety concepts
Patients undergoing acute care in healthcare establishments (such as hospitals) require enhanced reliability and safety of the electrical installation as well as the safe and reliable operation of the medical electrical (ME) equipment used. This is to provide security of supplies and minimize the risk of electric shock.
In medical locations, the risk to patients is increased due to:
- the reduction in body resistance, since the skin is often cut or broken or their defensive capacity is either reduced by medication or nullified while anaesthetized. These conditions increase the possible consequence of an electric shock under fault condition.
- the threat to the safety of the patient from failure of the supply especially to life-supporting equipment.
The presence of liquids such as blood and saline solutions will also add to the risk, because of the increased conductive area and subsequent lower-body resistance. The prolonged loss of the mains supply to life-supporting ME equipment may put the patient's life at risk. This equipment must have secure supplies to provide adequate safety.
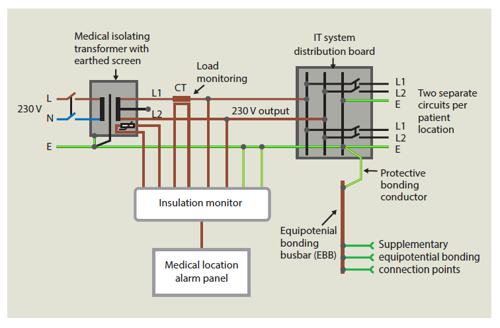
The introduction of medical IT systems has eliminated the operational hazards listed above. Earth fault currents in a medical IT system depend only on the capacitive coupling between the line conductors and earth. As these capacitances are quite low in value, the resultant capacitive coupling current is quite low, i.e. not dangerous. Similarly, a single earth fault will not cause a trip, it just creates a reference to earth (as in a TN system) so continuity of supply is present.
Shock hazards due to bodily contact with both AC and DC electric currents are well known and documented in IEC/TR2 60479-1 Effects of current on human beings and livestock – General aspects. This Technical Report indicates that, depending on skin resistance, path and area of contact, the impedance of the human body, environmental conditions and duration, currents of the order of 10 mA passing through the human body can result in muscular paralysis followed by respiratory paralysis. Eventual ventricular fibrillation can occur at currents just exceeding 20 mA.
In a normal environment, the average body impedance may be assumed to be 2 kW but in a medical location, where ME equipment is being used, the value given in BS EN 60601-1 (Medical electrical equipment – part 1: general requirements for basic safety and essential performance) is only 1 kW. Therefore, the risk in medical locations is increased, which is why the conventional touch voltage in medical locations of Group 1 and Group 2 is reduced from 50 V AC to 25 V AC.
Currents of the order of 10 µA have a probability of 0.2 % for causing ventricular fibrillation or pump failure when applied through a small area of the heart. At 50 µA, the probability of ventricular fibrillation increases to the order of 1 % (BS EN 60601-1).
Historical background
Since the early 1920s, appropriate safety measures for electrical installations in medical locations have been accorded considerable importance throughout the world.
International development of unearthed power supply (IT) systems in medical locations
The use of unearthed power supply systems (isolated terra – IT) in hospitals commenced in the USA between 1920 and 1930. During this period some very high-profile cases involving explosions and fires in operating theatres, in which combustible anaesthetic gases where used, such as cyclopropane and anaesthetic Ether. In 1939, this prompted the National Fire Protection Association (NFPA) to begin to develop guidelines with regard to the safe use of electrical engineering in hospitals. The first Regulation was published in the USA in 1944 Safe Practice in Hospital Operating Rooms. Further guidelines, NFPA 56, were published in 1949, recommending the use of unearthed power supply systems in all rooms where combustible gases are present. This then became the basis of NFPA 99, ‘The American Standard’. In 1959, the NFPA Standard was incorporated into the National Electric Code (NEC). Further developments of this Standard took place resulting in the publication of NFPA 70 National Electrical Code – NEC in 2017. Within this code, Article 517 covers the requirements for Healthcare Facilities.
As in all IT systems, an insulation monitoring device (IMD) is incorporated to indicate a first earth fault. Within the USA, this function is carried out by a line isolation monitor (LIM).
During this period of development, other countries were looking at other safety aspects, not only fires and explosions, but the risk of electric shock and continuity of supplies.
The Canadian Standard Association (CSA) published Standard Z 32 in 1963 under the title Code for Prevention of Explosion and Electric Shock in Hospital Operation Rooms. By 1970, this Code specified the installation of unearthed power supply systems for all “anaesthetizing locations”.
In Germany specifications for electrical installations in hospitals (DIN VDE 0170) were first published in 1962. An unearthed system with appropriate insulation monitoring devices (IMD) were recommended for certain rooms such as operating theatres and intensive care units.
In Italy, the Associazione Elettrotecnica Italiana (AEI) introduced a standard Impianti elettici in locali adibiti – (Electrical installations in locations for medical use). This standard recommended the deployment of unearthed power supply systems, including monitoring, in operating theatres.
In the Netherlands, unearthed power supply systems were introduced in 1975 under the standard NEN 3134 The Guide for Electrical Installation in Medically Used Rooms. This called for these systems to be installed in operating theatres and other surgical rooms. It stipulated that the power rating of a transformer should not exceed 1.6 kVA and the leakage current to not exceed 35 µA. Special requirements were imposed on the monitoring device.
In Australia, Code AS 3033 was published in 1976, Rules for Electrical Wiring and Equipment in Electromedical Treatment areas of Hospitals. Here again, the unearthed power system with appropriate monitoring devices for the overall impedance of the system with ELCB and load monitoring of the transformers were also specified.
Spain published its standard UNE 20-615-788 Systems with isolating transformers for medical applications and control and protective equipment associated therewith in 1978.
Similar recommendations for unearthed power supplies with monitoring was introduced in Chile a year later, NSEG 4.E.P Power supply for medically used rooms and in Japan in 1982.
The Electrotechnical Council of Ireland published the standard Technical Committee (TC 10) six years later. As well as the unearthed system, special emphasis was placed on equipotential bonding and the use ECLB.
Switzerland followed by publishing its installation standard for medically used areas, Med 4818/10.89 Regulation for electrical installation in medically used rooms. It contained a clear explanation of the unearthed power supply system and resistive insulation monitoring.
All the above codes and standards for medical locations initially followed the recognised recommendations for sub-dividing the medical rooms into groupings (G0, G1 and G2) depending on the type of activity and any requirement where continuity of supply is essential in case of a first fault. Also, do note that the prevention of electric shock in G2 locations is the most onerous. Refer to BS 7671:2018 for definitions of these groupings. All countries standards recommended using an IT transformer with associated insulation monitoring for G2 locations to supply life-support ME equipment.
Throughout the years mentioned above, the requirements for medical transformers and insulation monitors were not specified in the international standard, so each country listed its own requirements for transformer ratings and leakage currents including temperature/load monitoring. These requirements varied from 1.6 kVA in the Netherlands to 10 kVA in the USA. Transformer leakage currents varied from 15 µA in Canada, 100 µA in Japan and 500 µA in France and Germany. Transformer temperature/load monitoring were recommended by Germany and Australia only.
Development of the IEC Regulations for medical locations
In the mid-1980s, the IEC proposed a unified international standard to cover installations in Medical Locations. This task was first offered to IEC TC 62 (Electrical equipment in medical practice). This was passed onto Sub Committee, SC 62A (Common aspects of electrical equipment used in medical practice), in particular, Working Group (WG) 2. After some discussions, both at national and international levels, this project, having been recognised as an installation standard, was passed on to TC 64 (Electrical installations and protection against electric shock). A new WG (26) was formed under IEC TC 64 to continue with this task. The initial title of the proposed document was Electrical Installations in Medical Locations and Associated Areas and later on to Medical Locations. On completion, it was to be incorporated in Part 7, Special Locations (Section 710) of IEC 364 (currently IEC 60364).
Subsequently, international standards for the medical IT transformer and associated IMDs were developed and published, these are titled:
IEC 61558-2-15 (BS EN 61558-2-15: 2012) Particular requirements and tests for isolating transformers for the supply of medical locations and IEC 61557-8 (BS EN 61557-8: 2015), Annex A and Annex B (MED-IMD Medical insulation monitoring devices for IT systems).
The standard for medical IT transformers unified the requirements for its rating (0.5 kVA to 10 kVA) and its leakage current not to exceed 0.5 mA. Overload monitoring is required and achieved by incorporating an over-temperature monitor. Various other requirements such as rated frequency, supply voltage, inrush current and voltage regulation were also specified. It is interesting to note that the 0.5 mA leakage current is equivalent to types B and BF patient leakage currents of medical electrical equipment (BS EN 60601-1), under single fault condition (SFC).
Introduction of the Medical IT system to the UK
Within the UK, guidance on electrical installations in hospitals (healthcare facilities) had been published by the Department of Health (DoH) in various publications such as Health Guidance Notes (HGN) and Health Technical Memorandums (HTMs). In particular in HTM 7 circa 1960s Electrical Services Supply and Distribution. This HTM was updated to HTM 2007 (circa 1996). Two further updates took place, HTM 06-1 (circa 2007) and HTM 06-01 (circa 2017).
As the international development of the standard for installations in medical locations was assigned to IEC TC 64, the logical progression for this work within the UK was assigned to JPEL/64 in the late 1980s.
In 1988, the IEE (later the IET) approached the chief engineer at the DoH, requesting a nominee from the Department to join WG 26 at IEC level. The undersigned was nominated by the DoH and joined IEC TC 64 WG 26 in 1988.
In the IEE Wiring Regulations Sixteenth Edition (BS 7671:1992), Part 6 was assigned to “Special Installations or Locations” and Part 7 assigned to “Inspection and Testing”. Section 610 of Part 6 was reserved for future use, i.e. for “Medical Locations”. Later Part 6 was aligned with the IEC/CENELEC numbering system and Medical Locations became Section 710.
WG 26 of IEC TC 64 commenced drafting Section 710 in 1988 (over 31 years ago). Various Committee Drafts (CDs) were published over the next 14 years. Eventually, the first IEC Standard (IEC 60364-7-710) for Medical Locations was published in 2002.
The first time the DoH made a brief reference to special requirements for medical locations was in HTM 2007 (circa 1996) based on one of the CDs of WG 26. At IEE level, the first publication to include a reference to medical locations was in Guidance Note 7, Special Locations, Chapter 10 published in 1998.
Part of the discussions at WG 26 focused on the allocation of loading associated with various Group 2 locations to assist with the sizing of the medical IT transformer. This was to be included in an Informative Annex:
Allocation of recommended loads associated with Group 2 locations
|
Group 2 location |
Recommended loads |
|
Thoracic Theatre |
5.5 kVA / Theatre |
|
Cardiac Theatre |
7.5 kVA / Theatre |
|
Intensive Care Unit |
3 kVA / Bed |
|
Recovery Room |
2.5 kVA / Bed |
|
Paediatric ITU (ICU) |
3 kVA / Bed |
|
Ear Nose and Throat (ENT) Theatre |
5.5 kVA / Theatre |
|
Neonatal Unit |
3 kVA / Bed |
|
Paediatric Theatre |
5.5 kVA / Theatre |
However, this was omitted as different countries applied their own individual requirements.
It took another 10 years of discussions when the CENELEC (HD) Standard was published in 2012. The Medical Locations Standard (Section 710) was first incorporated in the IET Wiring Regulations, BS 7671:2008+A1:2011.
Currently, these standards, (IEC 60364-7-710/CLC 60364-7-710) are being revised and updated at international level with a possible publishing date marked for some time in 2020.
M. AL-RUFAIE B.Sc. (Hons.), C.Eng., MIET, FIHEEM
Medical Electrical Consultant
Member of JPEL/64D
